Pure liquids have molecules further apart from each other. Although molecules move about within the liquid, they are slow to pass heat to other regions compared to the free electrons in metals. This is because there are large inter-molecular distances between liquid molecules. There are also fewer and rare collisions between the molecules.
Why liquids are poor conductors of Heat
Admin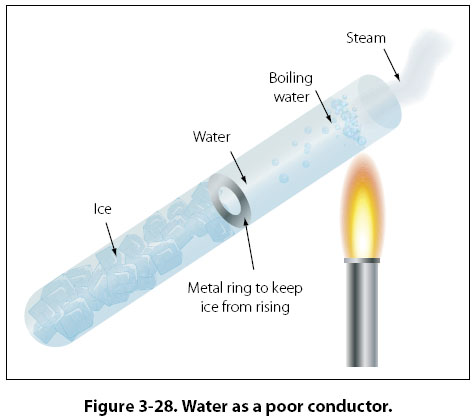

![[Explained] Newton’s Third Law](https://teachersofphysics.com/wp-content/uploads/2020/09/fall_2013_sketcheskey_3.jpg)